Unexpected binuclear O–O cleavage and radical C–H activation mechanism for Cu-catalyzed desaturation of lactone†
Abstract
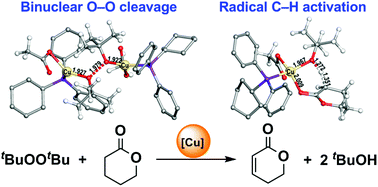
A density functional theory study of Cu-catalyzed desaturation of δ-valerolactone into α,β-unsaturated counterparts reveals an unexpected binuclear di-tert-butyl peroxide (DTBP) homolysis with spin-crossover and a radical α-C–H bond activation mechanism. The rate-determining step in the reaction catalyzed by CuIOAc-CyPPh2 is the homolysis of the O–O bond in DTBP with a total free energy barrier of 26.9 kcal mol−1, which is consistent with the observed first-order dependences on LCuI-PR3 and DTBP, as well as the pseudo-zeroth-order with lactone. The α- and β-H transfer steps have 0.3 and 14.8 kcal mol−1 lower barriers than the O–O cleavage process, respectively. Such different barriers well explain the observed weak kinetic isotopic effect (KIE) at α-H and no KIE at β-H. In addition, we found that the replacement of CyPPh2 for pyridine in the Cu complexes leads to much higher barriers for O–O bond cleavage and C–H bond activations with the formation of more stable binuclear Cu complexes.


 Please wait while we load your content...
Please wait while we load your content...