Mechanism of nickel-catalyzed direct carbonyl-Heck coupling reaction: the crucial role of second-sphere interactions†
Abstract
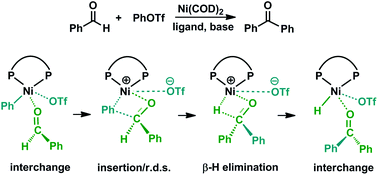
We present a detailed DFT mechanistic study on the first Ni-catalyzed direct carbonyl-Heck coupling of aryl triflates and aldehydes to afford ketones. The precatalyst Ni(COD)2 is activated with the phosphine (phos) ligand, followed by coordination of the substrate PhOTf, to form [Ni(phos)(PhOTf)] for intramolecular PhOTf to Ni(0) oxidative addition. The ensuing phenyl-Ni(II) triflate complex substitutes benzaldehyde for triflate by an interchange mechanism, leaving the triflate anion in the second coordination sphere held by Coulomb attraction. The Ni(II) complex cation undergoes benzaldehyde C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O insertion into the Ni–Ph bond, followed by β-hydride elimination, to produce Ni(II)-bound benzophenone, which is released by interchange with triflate. The resulting neutral Ni(II) hydride complex leads to regeneration of the active catalyst following base-mediated deprotonation/reduction. The benzaldehyde C
O insertion into the Ni–Ph bond, followed by β-hydride elimination, to produce Ni(II)-bound benzophenone, which is released by interchange with triflate. The resulting neutral Ni(II) hydride complex leads to regeneration of the active catalyst following base-mediated deprotonation/reduction. The benzaldehyde C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O insertion is the rate-determining step. The triflate anion, while remaining in the second sphere, engages in electrostatic interactions with the first sphere, thereby stabilizing the intermediate/transition state and enabling the desired reactivity. This is the first time that such second-sphere interaction and its impact on cross-coupling reactivity has been elucidated. The new insights gained from this study can help better understand and improve Heck-type reactions.
O insertion is the rate-determining step. The triflate anion, while remaining in the second sphere, engages in electrostatic interactions with the first sphere, thereby stabilizing the intermediate/transition state and enabling the desired reactivity. This is the first time that such second-sphere interaction and its impact on cross-coupling reactivity has been elucidated. The new insights gained from this study can help better understand and improve Heck-type reactions.


 Please wait while we load your content...
Please wait while we load your content...