Exploring the hyperpolarisation of EGTA-based ligands using SABRE†
Abstract
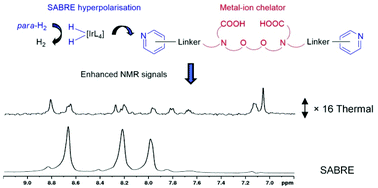
The design of molecules whose magnetic resonance (MR) signals report on their biological environment is receiving attention as a route to non-invasive functional MR. Hyperpolarisation techniques improve the sensitivity of MR and enable real time low concentration MR imaging, allowing for the development of novel functional imaging methodologies. In this work, we report on the synthesis of a series of EGTA-derived molecules (EGTA – ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), whose core structures are known to bind biologically relevant metal ions in vivo, in addition to pyridyl rings that allow reversible ligation to an iridium dihydride complex. Consequently, they are amenable to hyperpolarisation through the parahydrogen-based signal amplification by reversible exchange (SABRE) process. We investigate how the proximity of EGTA and pyridine units, and the identity of the linker group, affect the SABRE hyperpolarisation attained for each agent. We also describe the effect of catalyst identity and co-ligand presence on these measurements and can achieve 1H NMR signal enhancements of up to 160-fold. We rationalise these results to suggest the design elements needed for probes amenable to SABRE hyperpolarisation whose MR signals might in the future report on the presence of metal ions.


 Please wait while we load your content...
Please wait while we load your content...