Copper(ii) complexes of N-propargyl cyclam ligands reveal a range of coordination modes and colours, and unexpected reactivity†
Abstract
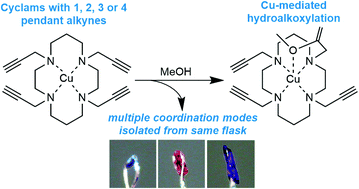
The coordination chemistry of N-functionalised cyclam ligands has a rich history, yet cyclam derivatives with pendant alkynes are largely unexplored. This is despite the significant potential and burgeoning application of N-propargyl cyclams and related compounds in the creation of diversely functionalised cyclam derivatives via copper-catalysed azide–alkyne ‘click’ reactions. Herein we describe single crystal X-ray diffraction and spectroscopic investigations of the coordination chemistry of copper(II) complexes of cyclam derivatives with between 1 and 4 pendant alkynes. The crystal structures of these copper complexes unexpectedly reveal a range of coordination modes, and the surprising occurrence of five unique complexes within a single recrystallisation of the tetra-N-propargyl cyclam ligand. One of these species exhibits weak intramolecular copper-alkyne coordination, and another is formed by a surprising intramolecular copper-mediated hydroalkoxylation reaction with the solvent methanol, transforming one of the pendant alkynes to an enol ether. Multiple functionalisation of the tetra-N-propargyl ligand is demonstrated via a ‘tetra-click’ reaction with benzyl azide, and the copper-binding behaviour of the resulting tetra-triazole ligand is characterised spectroscopically.
- This article is part of the themed collection: Bioorthogonal and click chemistry: Celebrating the 2022 Nobel Prize in Chemistry


 Please wait while we load your content...
Please wait while we load your content...