Aerobic oxidation of primary amines to amides catalyzed by an annulated mesoionic carbene (MIC) stabilized Ru complex†
Abstract
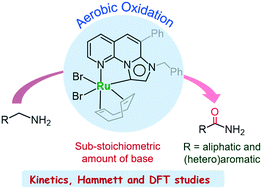
Catalytic aerobic oxidation of primary amines to the amides, using the precatalyst [Ru(COD)(L1)Br2] (1) bearing an annulated π-conjugated imidazo[1,2-a][1,8]naphthyridine-based mesoionic carbene ligand L1, is disclosed. This catalytic protocol is distinguished by its high activity and selectivity, wide substrate scope and modest reaction conditions. A variety of primary amines, RCH2NH2 (R = aliphatic, aromatic and heteroaromatic), are converted to the corresponding amides using ambient air as an oxidant in the presence of a sub-stoichiometric amount of KOtBu in tBuOH. A set of control experiments, Hammett relationships, kinetic studies and DFT calculations are undertaken to divulge mechanistic details of the amine oxidation using 1. The catalytic reaction involves abstraction of two amine protons and two benzylic hydrogen atoms of the metal–bound primary amine by the oxo and hydroxo ligands, respectively. A β-hydride transfer step for the benzylic C–H bond cleavage is not supported by Hammett studies. The nitrile generated by the catalytic oxidation undergoes hydration to afford the amide as the final product.


 Please wait while we load your content...
Please wait while we load your content...