Mechanistic insights into carbamate formation from CO2 and amines: the role of guanidine–CO2 adducts†
Abstract
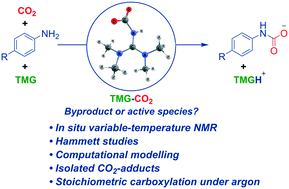
Capture of CO2 by amines is an attractive synthetic strategy for the formation of carbamates. Such reactions can be mediated by superbases, such as 1,1,3,3-tetramethylguanidine (TMG), with previous implications that zwitterionic superbase–CO2 adducts are able to actively transfer the carboxylate group to various substrates. Here we report a detailed investigation of zwitterionic TMG–CO2, including isolation, NMR behavior, reactivity, and mechanistic consequences in carboxylation of aniline-derivatives. Our computational and experimental mechanistic analysis shows that the reversible TMG–CO2 zwitterion is not a direct carboxylation agent. Instead, CO2 dissociates from TMG–CO2 before a concerted carboxylation occurs, where the role of the TMG is to deprotonate the amine as it is attacking a free CO2. This insight is significant, as it opens a rational way to design new synthesis strategies. As shown here, nucleophiles otherwise inert towards CO2 can be carboxylated, even without a CO2 atmosphere, using TMG–CO2 as a stoichiometric source of CO2. We also show that natural abundance 15N NMR is sensitive for zwitterion formation, complementing variable-temperature NMR studies.


 Please wait while we load your content...
Please wait while we load your content...