Conversion of methane to acetonitrile over GaN catalysts derived from gallium nitrate hydrate co-pyrolyzed with melamine, melem, or g-C3N4: the influence of nitrogen precursors†
Abstract
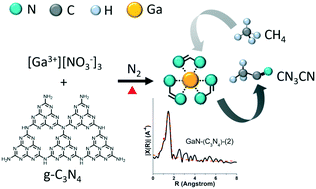
Co-pyrolyzing gallium nitrate hydrate and melamine, melem, or g-C3N4 generates gallium nitride (GaN) for the conversion of methane to acetonitrile (AcCN). The solid-state-pyrolysis-made GaN catalysts exhibited better activity than commercial GaN. Among the as-prepared catalysts, GaN made by using g-C3N4 with a N/Ga ratio of 2 (i.e., GaN-(C3N4)-(2)) was the most attractive: a high initial methane conversion (28.2%), a high initial AcCN productivity (151 μmol gcat−1 min−1), and a 6 h accumulated AcCN yield (5816 μmol gcat−1) were obtained at 700 °C with a space time of 3000 mLCH4 gcat−1 h−1. GaN-(C3N4)-(2) had finely dispersed GaN crystals and enriched amorphous CN species (e.g., sp2 N and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups), and both are important in promoting the methane conversion rate. GaN agglomeration, coke deposition, and depleted CN species contributed to the deactivation of the catalyst, and a nitridation–activation process could rejuvenate the activity partially. The analysis of the structure–activity correlation revealed that the accumulated AcCN yield had an inverse trend with respect to the crystallite size of GaN and the sp3/sp2 ratio of the N environment.
N groups), and both are important in promoting the methane conversion rate. GaN agglomeration, coke deposition, and depleted CN species contributed to the deactivation of the catalyst, and a nitridation–activation process could rejuvenate the activity partially. The analysis of the structure–activity correlation revealed that the accumulated AcCN yield had an inverse trend with respect to the crystallite size of GaN and the sp3/sp2 ratio of the N environment.


 Please wait while we load your content...
Please wait while we load your content...