The fate of oxygen on graphene-catalyst in the photocatalytic water splitting reaction†
Abstract
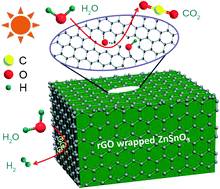
Graphene has been extensively used in photocatalytic water splitting for enhancing the separation of photogenerated electrons and holes and/or introducing intermediate levels in the band gap to obtain visible-light absorption. However, with a hydrogen uptake, a fundamental understanding of the simultaneous transformation process of oxygen on graphene has always been neglected in most research. To illustrate this important problem, an only 3 layered graphene-exposed photocatalyst (hollow perovskite ZnSnO3-reduced graphene oxide (rGO) nanocubes) is skillfully constructed for the first time, in which ∼4 nm ZnSnO3 nanoparticle aggregates are closely wrapped up by rGO with C–O–Zn (Sn) chemical bonds connection. Assisted by this ideal robust model without the effect of externally exposed metal oxides, the transformation process of oxygen in water splitting is performed at the C vacancy defect in graphene, which is clarified through in situ Raman spectroscopy and gas chromatography analysis. It is also found that the oxygen of H2O on rGO undergoes a step-by-step dehydrogenation process from the intermediate OH–C and O–C to the final CO2. This research may pave the way for future photocatalytic overall water splitting technologies based on graphene.


 Please wait while we load your content...
Please wait while we load your content...