Photocontrolled lactide ROP by the light-regulated release of potassium acetate from an azobenzene-bridged crown ether†
Abstract
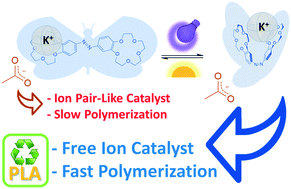
An azobenzene-bridged 15-crown-5 ether (1) was successfully used as a photoswitchable cocatalyst for modulating the catalytic activity of potassium acetate (KOAc) in the ring-opening polymerization (ROP) of L-lactide (L-LA) when initiated from an exogenous alcohol. By alternating exposure to daylight and UV light, 1 transforms the K+,−OAc ion pair-like in free ions “on-demand”, resulting in an effective form of the catalytic complex. This allows the modulation of the L-LA ROP speed, switching the process from a slow to a fast state.


 Please wait while we load your content...
Please wait while we load your content...