Origins of Lewis acid acceleration in nickel-catalysed C–H, C–C and C–O bond cleavage†
Abstract
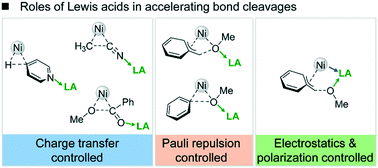
The current understanding of Lewis acid effects on transition metal catalysis is generally based on the enhanced charge transfer from metal to substrate due to the formation of Lewis acid–base adducts. The critical factors of how Lewis acids manipulate complex catalyst–substrate interactions to facilitate reactions are seldom clarified. Herein, using the energy decomposition approach, we quantify the contributions of multiple factors which account for the Lewis acid acceleration in Ni-catalyzed C–X (X = H, C, O) bond cleavage via oxidative addition. The results reveal that the dominant factors for Lewis acid promotion highly depend on the features of transition states with Lewis acids. In the transition states having only heteroatom–Lewis acid interactions (e.g., C–H, C–CN and C(acyl)–O oxidative additions), the reactivity is improved majorly by enhancing charge transfer from the metal to the Lewis acid-activated substrates, which is consistent with the conventional viewpoint. However, for the transition states with heteroatom–Lewis acid and heteroatom–transition metal interactions (e.g., C(benzyl)–O and C(aryl)–O oxidative additions), the decisive factor for the improved reactivity is ascribed to the reduced Pauli repulsion between occupied orbitals. Further, in the transition states having heteroatom–Lewis acid and Lewis acid–transition metal interactions (e.g., C(benzyl)–O oxidative addition), the reaction is facilitated by strengthening electrostatics and polarization due to greater charge separation and electron delocalization effects. These three types of dominant factors are generally employed by a series of different Lewis acids in promoting Ni-catalyzed bond cleavage.


 Please wait while we load your content...
Please wait while we load your content...