Kinetic study and molecular dynamics simulation of two novel mannose isomerases†
Abstract
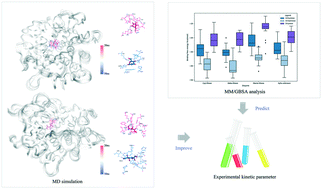
D-Mannose can be used as a nutritional supplement due to its physiological functions. It can be directly converted from abundant D-fructose using mannose isomerase (MIase) as a biocatalyst. In this study, two novel MIases were heterologously expressed and characterized. The MIases displayed the highest specific activity towards D-mannose among the tested substrates, followed by D-fructose and D-lyxose. Their maximum enzyme activities exhibited at 55 and 50 °C under a neutral environment. We performed molecular dynamics (MD) simulation and binding free energy calculation to study the substrate binding process of MIase. The computational work provides insight into the binding manners of D-mannose in MIases. The substrate in MIases may undergo a transition after the ring opening. The calculated relative binding free energy was in line with the experimentally obtained kinetic parameters. The substrate affinity of the MIases follows the order: D-mannose > D-fructose > D-lyxose.


 Please wait while we load your content...
Please wait while we load your content...