Heterodinuclear catalysts Zn(ii)/M and Mg(ii)/M, where M = Na(i), Ca(ii) or Cd(ii), for phthalic anhydride/cyclohexene oxide ring opening copolymerisation†
Abstract
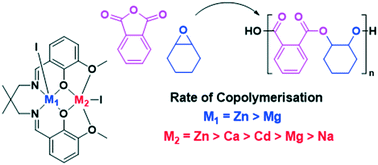
Anhydride and epoxide ring opening copolymerisation is a useful and controlled route to aliphatic, semi-aromatic and functionalised polyesters. Building upon our prior investigations of synergic Zn(II)/Mg(II) and Mg(II)/Co(II) polymerisation catalysts, here, a series of heterodinuclear catalysts for phthalic anhydride/cyclohexene oxide ROCOP are reported. The complexes feature either Zn(II)/M or Mg(II)/M combinations, where M = Na(I), Ca(II) or Cd(II), and are coordinated by an ‘open’ dinucleating ortho-vanillin derived Schiff-base ligand. The ligand features two binding ‘pockets’: 1) a coordination site comprising diphenolate and dimine moieties and 2) a site featuring diphenolate and diether groups. Heterodinuclear complex syntheses are high yielding due in part to the different coordination chemistries of the binding ‘pockets’. All new complexes are fully characterised including by multi-nuclear NMR spectroscopy and X-ray diffraction experiments; several solid state structures are Lewis base adducts with the crystallisation solvents (THF or acetonitrile) and provide insights into the structures of catalytic cycle intermediates. The catalysts show good performances and quantitative formation of ester linkages. They show equivalent activity to the widely investigated [(salen)Cr(III)Cl]/PPNCl systems (TOF = 67 h−1, 0.1 mol%, 100 °C, 17.6 kg mol−1) but lower activity than previously reported Zn(II)/Mg(II) catalysts or than tethered metal-salen catalysts. All the complexes are less active than the Zn(II)/Zn(II) homodinuclear complex proving that catalytic synergy is not provided only by heterodinuclear complexes with different functions; these findings underscore the need for continued exploration of the factors underpinning synergic heterometallic combinations.


 Please wait while we load your content...
Please wait while we load your content...