Dehydropolymerisation of methylamine borane using highly active rhodium(iii) bis(thiophosphinite) pincer complexes: catalytic and mechanistic insights†‡
Abstract
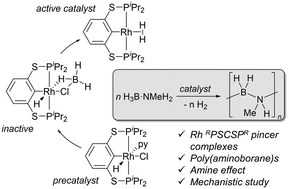
The bis(thiophosphinite) pincer complexes [(RPSCSPR)Rh(py)(H)(Cl)] (RPSCSPR = C6H4–2,6-(SPR2)2 with R = iPr, 2a and R = Ph, 2b) are prepared by metalation of the ligand precursor with [Rh(cod)Cl]2 in the presence of pyridine. Both complexes are highly active for the dehydropolymerisation of H3B·NMeH2, producing poly(aminoborane)s (H2BNMeH)n (e.g. 1 mol% 2a, Mn = 20.200 g mol−1) in very short reaction times at room temperature. Detailed NMR studies on the iPr substituted complex 2a reveal B–N bond cleavage as a dominant process, resulting in the initial formation of a BH3 adduct, which inhibits catalysis, as evidenced by a pronounced induction period. This can be removed by addition of amine to ultimately bring the catalyst on-cycle for dehydrogenation and polymer formation. A mechanistic scenario is discussed based on the volumetric, spectroscopic and size-exclusion chromatography data as well as M06L-SCRF density functional theory computations.


 Please wait while we load your content...
Please wait while we load your content...