Cyclopentadienone–NHC iron(0) complexes as low valent electrocatalysts for water oxidation†
Abstract
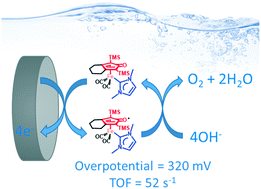
The design and development of stable molecular iron electrocatalysts able to work with low overpotential in the oxidation of water to molecular oxygen is an essential challenge for sustainable energy applications. Our group has recently developed stable iron(0) N-heterocyclic carbene (NHC) complexes bearing a non-innocent cyclopentadienone (Cp![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ligand. This peculiar ligands combination is herein exploited to tune the electrochemistry of the corresponding complexes: NHCs regulate the anodic process in a suitable potential region for oxidation of water to O2 and cyclopentadienone promotes a mono-electronic redox process through the formation of a radical complex (1˙). The synergic effect of these ligands on iron complexes makes them suitable, for the first time, as low valent electrocatalysts for water oxidation. The electrocatalytic activity was determined in water/THF with added KOH. Complex 1 shows the best catalytic activity and competitive efficiency in terms of both TOF (up to 52 s−1) and overpotential (320 mV).
O) ligand. This peculiar ligands combination is herein exploited to tune the electrochemistry of the corresponding complexes: NHCs regulate the anodic process in a suitable potential region for oxidation of water to O2 and cyclopentadienone promotes a mono-electronic redox process through the formation of a radical complex (1˙). The synergic effect of these ligands on iron complexes makes them suitable, for the first time, as low valent electrocatalysts for water oxidation. The electrocatalytic activity was determined in water/THF with added KOH. Complex 1 shows the best catalytic activity and competitive efficiency in terms of both TOF (up to 52 s−1) and overpotential (320 mV).


 Please wait while we load your content...
Please wait while we load your content...