Ethanol conversion over Ga2O3–ZrO2 solid solution: empirical evidence of the reaction pathway, the surface acid–base properties, and the role of gallium ions†
Abstract
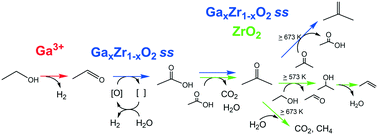
Ethanol conversion over Ga2O3–ZrO2 solid solution was examined in the temperature range of 573–773 K, and acetone/isobutene formation was confirmed under cofeeding of H2O vapor. The reaction pathway was empirically investigated with the contact time dependency and reactivity of the five possible intermediates. The surface acid–base properties were discussed with 1-butene isomerization, 2-butanol decomposition and the poisoning experiment, the dehydration/dehydrogenation selectivity of C3–C4 primary and secondary alcohols, and CO2-TPD. The Ga2O3–ZrO2 catalyst was less basic than ZrO2 itself and exhibited a stronger dehydrogenation property. Alcohol dehydrogenation over Ga2O3–ZrO2 was suggested to be promoted by the specific dehydrogenation property of isolated Ga3+ species. Acetaldehyde formed by ethanol dehydrogenation was converted into acetone, mainly via acetate species in the presence of H2O. Coexistence of ZrO2, doped elements with dehydrogenation capability, surface acid–base properties, and H2O vapor is important for ethanol to acetone/isobutene reaction.


 Please wait while we load your content...
Please wait while we load your content...