Lewis pairing and frustration of group 13/15 elements geometrically enforced by (ace)naphthalene, biphenylene and (thio)xanthene backbones†
Abstract
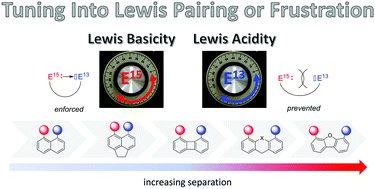
The synthesis, structure, and reactivity of mixed group 13/group 15 compounds (E13 = B, Al, Ga, In, Tl; E15 = N, P, Sb, Bi) featuring a rigid (ace)naphthalene or (thio)xanthene backbone are discussed in this review. The backbone may either enforce or prevent E15→E13 interactions, resulting in Lewis pairing or frustration. The formation of strong E15→E13 interactions is possible upon peri-substitution of (ace)naphthalenes. This gives the opportunity to access and study highly reactive species, as exemplified by P-stabilised borenium salts and boryl radicals. In turn, rigid expanded spacers such as biphenylenes, (thio)xanthenes and dibenzofurans impose long distances and geometrically prevent E15→E13 interactions. Such P–B derivatives display ambiphilic coordination properties and frustrated Lewis pair behaviour towards small molecules, their preorganised structure favouring reversible interaction/activation. Throughout the review, the importance of the scaffold in enforcing or preventing E15→E13 interactions is highlighted and discussed based on experimental data and theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...