Guest-occupiable space in the crystalline solid state: a simple rule-of-thumb for predicting occupancy†
Abstract
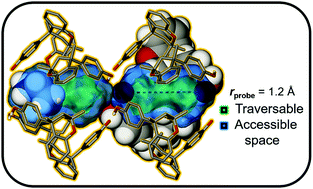
The generally greater degree of thermal motion of guest molecule(s) relative to the host often impedes their accurate modelling in crystal structures. We propose a ‘rule-of-thumb’ for estimating the maximum number of guest molecules that can be accommodated in a given amount of accessible space in an adequately modelled host structure. A survey of the Cambridge Structural Database was carried out to evaluate the fractional occupancy θ of the accessible space for almost 40 000 solvates involving 20 common solvents. Using widely accessible software tools, the volume of a guest is estimated as its van der Waals surface, while the guest-occupiable space of a potentially porous host is determined as that available to a virtual spherical probe. We propose terminology more appropriate to the supramolecular interpretation of surface typology: the probe-traversable and probe-accessible boundaries as traced out by the locus and surface of a spherical probe, respectively. High-throughput analysis using commercial and free software packages yielded a mean θ = 51.1(4)%, ranging from 45.3(6)% for hexane to 60(1)% for acetic acid.


 Please wait while we load your content...
Please wait while we load your content...