Reactivity of ynamides in catalytic intermolecular annulations
Abstract
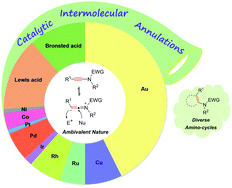
Ynamides are unique alkynes with a carbon–carbon triple bond directly attached to the nitrogen atom bearing an electron-withdrawing group. The alkyne is strongly polarized by the electron-donating nitrogen atom, but its high reactivity can be finely tempered by the electron-withdrawing group. Accordingly, ynamides are endowed with both nucleophilic and electrophilic properties and their chemistry has been an active research field. The catalytic intermolecular annulations of ynamides, featuring divergent assembly of structurally important amino-heterocycles in a regioselective manner, have gained much attention over the past decade. This review aims to provide a comprehensive summary of the advances achieved in this area involving transition metal and acid catalysis. Moreover, the intermolecular annulations of ynamide analogs including ynol ethers and thioalkynes are also discussed, which can provide insights into the reactivity difference caused by the heteroatoms.


 Please wait while we load your content...
Please wait while we load your content...