Origins of the photoinitiation capacity of aromatic thiols as photoinitiatiors: a computational study†
Abstract
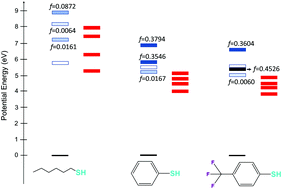
In this work, we report the photophysical properties of three thiol derivatives, commonly used as photoinitiators in thiol–ene free radical polymerization, the ultimate goal being to rationalize the main reason behind the photoinitiation efficiency. For this aim, time dependent density functional theory is used to simulate the absorption spectra of alkyl thiol (R–SH), thiophenol (PhSH) and p-(trifluoromethyl) thiophenol (p-CF3PhSH), describe their excited state topologies, and explore their potential energy surfaces along the S–H dissociation. Excited state calculations have shown that the S–H photolysis is achieved through the triplet excited states following intersystem crossing from the originally populated singlet manifolds. More specifically, while in aromatic thiol derivatives dissociation is mainly triplet-state mediated, the first excited singlet state and first triplet state of alkyl thiol are both dissociative and hence potentially capable of generating the photoinduced radical species. We have also justified the experimental findings concerning the photoinitiator efficiency considering both their potential energy surface topologies and the absorption intensity, in the lowest energy region.


 Please wait while we load your content...
Please wait while we load your content...