The effect of elastic strains on the adsorption energy of H, O, and OH in transition metals†
Abstract
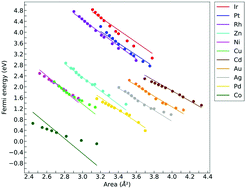
The influence of elastic strains on the adsorption of H, O, and OH on the (111) surfaces of 8 fcc (Ni, Cu, Pd, Ag, Pt, Au, Rh, Ir) and on the (0001) surfaces of 3 hcp (Co, Zn, Cd) transition metals was analyzed by means of density functional theory calculations. To this end, surface slabs were subjected to different strain states (uniaxial, biaxial, shear, and a combination of them) up to strains dictated by the mechanical stability limits indicated by phonon calculations. It was found that the adsorption energy followed the predictions of the d-band theory but – surprisingly – the variations in the adsorption energy only depended on the area of the adsorption hole and not on the particular elastic strain tensor applied to achieve this area. The analysis of the electronic structure showed that the applied strains did not modify the shape of the Projected Density of States (PDOS) of the d-orbitals of the transition metals but only led to a shift in the energy levels. Moreover, the presence of the adsorbates on the surfaces led to negligible changes in the PDOS. Thus, the adsorption energies were a function of the Fermi energy which in turn was associated with the change of the area of the adsorption through a general linear law that was valid for all metals. The information in this paper allows the immediate and accurate estimation of the effect of any elastic strain on the adsorption energies of H, O, and OH in 11 transition metals with more than half-filled d-orbitals.


 Please wait while we load your content...
Please wait while we load your content...