Effects of water on the solvation and structure of lipase in deep eutectic solvents containing a protein destabilizer and stabilizer
Abstract
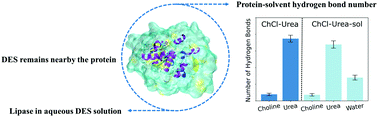
Aqueous deep eutectic solvent (DES) solutions emerge as new media for biocatalysis. The large number of DESs provides a space for designing solutions with desired features. One challenge for this design is to understand the fundamental relationship between the water effect on biocatalysis and the DES compositions. We investigate the solvation and structure of a lipase protein in two DESs containing a protein destabilizer (choline : urea (1 : 2)) and stabilizer (choline : glycerol (1 : 2)) and their 1 : 1 aqueous solution using molecular dynamics simulations. The lipase protein in the pure aqueous solution is simulated as the reference. The lipase protein remains folded in both DESs and their aqueous solutions. In both DESs, water molecules weaken the solvation shell of the lipase protein by reducing the protein-DES hydrogen bond lifetimes. However, the water molecules change the surface area and conformation of the active site on the lipase protein differently in the two DESs. Our simulations indicate that the impact on active sites plays an important role in differentiating the effect of water on biocatalysis in aqueous DESs.
- This article is part of the themed collection: Biocatalysis: A cross-journal collection


 Please wait while we load your content...
Please wait while we load your content...