Enzyme aggregation and fragmentation induced by catalysis relevant species†
Abstract
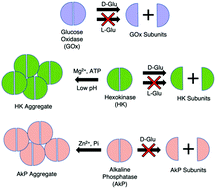
It is usually assumed that enzymes retain their native structure during catalysis. However, the aggregation and fragmentation of proteins can be difficult to detect and sometimes conclusions are drawn based on the assumption that the protein is in its native form. We have examined three model enzymes, alkaline phosphatase (AkP), hexokinase (HK) and glucose oxidase (GOx). We find that these enzymes aggregate or fragment after addition of chemical species directly related to their catalysis. We used several independent techniques to study this behavior. Specifically, we found that glucose oxidase and hexokinase fragment in the presence of D-glucose but not L-glucose, while hexokinase aggregates in the presence of Mg2+ ion and either ATP or ADP at low pH. Alkaline phosphatase aggregates in the presence of Zn2+ ion and inorganic phosphate. The aggregation of hexokinase and alkaline phosphatase does not appear to attenuate their catalytic activity. Our study indicates that specific multimeric structures of native enzymes may not be retained during catalysis and suggests pathways for different enzymes to associate or separate over the course of substrate turnover.


 Please wait while we load your content...
Please wait while we load your content...