Surface characterization and methane activation on SnOx/Cu2O/Cu(111) inverse oxide/metal catalysts†
Abstract
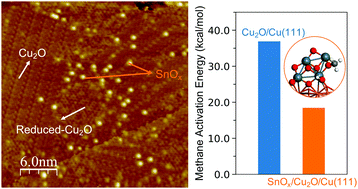
To activate methane at low or medium temperatures is a difficult task and a pre-requisite for the conversion of this light alkane into high value chemicals. Herein, we report the preparation and characterizations of novel SnOx/Cu2O/Cu(111) interfaces that enable low-temperature methane activation. Scanning tunneling microscopy identified small, well-dispersed SnOx nanoclusters on the Cu2O/Cu(111) substrate with an average size of 8 Å, and such morphology was sustained up to 450 K in UHV annealing. Ambient pressure X-ray photoelectron spectroscopy showed that hydrocarbon species (CHx groups), the product of methane activation, were formed on SnOx/Cu2O/Cu(111) at a temperature as low as 300 K. An essential role of the SnOx–Cu2O interface was evinced by the SnOx coverage dependence. Systems with a small amount of tin oxide, 0.1–0.2 ML coverage, produced the highest concentration of adsorbed CHx groups. Calculations based on density functional theory showed a drastic reduction in the activation barrier for C–H bond cleavage when going from Cu2O/Cu(111) to SnOx/Cu2O/Cu(111). On the supported SnOx, the dissociation of methane was highly exothermic (ΔE ∼ −35 kcal mol−1) and the calculated barrier for activation (∼20 kcal mol−1) could be overcome at 300–500 K, target temperatures for the conversion of methane to high value chemicals.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...