Crystal structures and superconductivity of lithium and fluorine implanted gold hydrides under high pressures†
Abstract
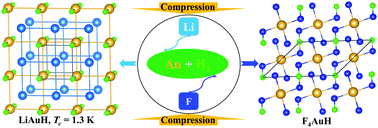
The investigations on gold science have been capturing research interest due to its diverse physical and chemical properties. Gold hydrides in the solid state, as a member of the Au compound family, are rare since the reaction of Au with H is hindered in terms of their similar electronegativity. It is expected that Li and F can provide electrons and holes, respectively, to help stabilize gold hydrides under high pressure. Herein, by means of a crystal structural search based on particle swarm optimization methodology accompanied by first-principles calculations, four hitherto unknown Li–Au–H compounds (i.e., LiAuH, LiAu2H, Li2Au2H, and Li6AuH) are predicted to be stable under compression. Intriguingly, Au–H bonding is found in LiAuH, LiAu2H, and Li2Au2H. As the gold content increases, Au atom arrangements exhibit diverse forms, from the chain in Li6AuH, the square layer in LiAuH, the network in Li2Au2H, and eventually to the coexistence of square and pyramid layers in LiAu2H. Additionally, Li6AuH has a unique cage-type lithium structure. Furthermore, electron–phonon coupling calculations show that these Li–Au–H phases are phonon-modulated superconductors with a superconducting critical temperature of 1.3, 0.06, and 0.02 K at 25 GPa and 2.79 K at 100 GPa. In contrast, we also identified two solid F4AuH and F6AuH phases with unexpected semiconductivity. They have structural configurations of H-bridged AuF4 quasi-square components and distorted AuF6 octahedrons, respectively, and have no gold-to-hydrogen bonds. Our current results indicate that electron doping at suitable concentrations under pressure can stabilize unique gold hydrides, and provide deep insights into the structures, electron properties, bonding behavior, and stability mechanism of ternary Li–Au–H and F–Au–H compounds.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...