Local structure and NO adsorption/desorption property of Pd2+ cations at different paired Al sites in CHA zeolite†
Abstract
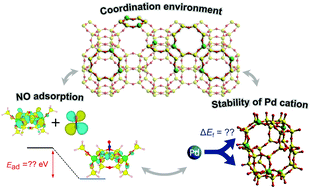
Recently, Pd-exchanged CHA zeolites (Pd-CHA) have attracted attention as promising passive NOx adsorbers (PNAs) for reducing NOx emissions during the cold start period of a vehicle engine. In this work, the relationship between the local structures and the NO adsorption/desorption properties of the Pd cations in CHA zeolites was investigated. Pd cation formation and NO adsorption were theoretically explored by density functional theory (DFT) calculations for different paired Al sites in six-/eight-membered rings (6MR/8MR). Furthermore, we prepared a series of Pd-CHAs with different Pd loadings (0.5–5.4 wt%) and evaluated their NO adsorption/desorption properties by in situ infrared (IR) spectroscopy and temperature-programmed desorption (TPD) measurements. The increase in the Pd loading resulted in a shift in the NO desorption temperature toward a higher temperature regime. This phenomenon was ascribed to the increase in the proportion of less stable Pd cations, resulting in improved NO adsorption. Furthermore, the effect of Al distribution on the NO adsorption property of Pd-CHA was examined using CHA zeolites containing different proportions of paired Al sites in 6MR while maintaining similar Si/Al ratios (Si/Al = 12.0–16.5). The present study, based on a combination of theoretical and experimental techniques, shows that the NO adsorption/desorption properties over Pd-CHA can be tuned by controlling the Pd loading amount and the type of paired Al sites.


 Please wait while we load your content...
Please wait while we load your content...