Kinetics of IO radicals with ethyl formate and ethyl acetate: a study using cavity ring-down spectroscopy and theoretical methods†
Abstract
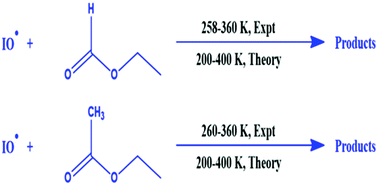
The gas-phase kinetics of the reactions of IO radicals with ethyl formate (EF) and ethyl acetate (EA) were investigated experimentally using cavity ring-down spectroscopy (CRDS). IO radicals were generated in situ in the CRD reaction zone by photolyzing a mixture of (CH3I + O3 + N2) at 248 nm and thereby probed at 445.04 nm. The rate coefficients for the reactions (IO + EF) and (IO + EA) were measured at a total pressure of 65 Torr of N2 in the temperature range of 258–358 and 260–360 K, respectively. The rate coefficients for the reactions (IO + EF) and (IO + EA) were measured experimentally at room temperature to be kExpt,298KIO+EF = (3.38 ± 0.67) × 10−14 and kExpt,298KIO+EA = (1.56 ± 0.30) × 10−13 cm3 molecule−1 s−1, respectively. The effects of pressure and photolysis laser fluence on the kinetics of test reactions were found to be negligible within the experimental uncertainties for the studied range. To complement our experimental findings, the kinetics of the title reactions were investigated theoretically using canonical variational transition state theory (CVT) with small curvature tunnelling (SCT) at the CCSD(T)//M06-2X/def2-SV(P) level of theory in temperatures between 200 and 400 K. Very good agreement was observed between the experimentally measured and theoretically calculated rate coefficients for both the reactions at 298 K. The thermochemical parameters as well as the branching ratios for the title reactions are also discussed in this study.


 Please wait while we load your content...
Please wait while we load your content...