α-Synuclein conformations followed by vibrational optical activity. Simulation and understanding of the spectra†
Abstract
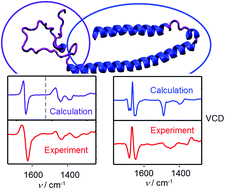
α-Synuclein is a neuronal protein which adopts multiple conformations. These can be conveniently studied by the spectroscopy of vibrational optical activity (VOA). However, the interpretation of VOA spectra based on quantum-chemical simulations is difficult. To overcome the hampering of the computations by the protein size, we used the Cartesian tensor transfer technique to investigate links between the spectral shapes and protein structure. Vibrational circular dichroism (VCD) and Raman optical activity (ROA) spectra of α-synuclein in disordered, α-helical and β-sheet (fibril) forms were measured and analyzed on the basis of molecular dynamics and density functional theory computations. For the disordered and α-helical conformers, a high fidelity of the simulated spectra with a reasonable computational cost was achieved. Most experimental spectral features could be assigned to the structure. So far unreported ROA marker bands of the secondary structure were found for the lower-frequency and CH stretching vibrations. Fibril VCD spectra were simulated with a rigid periodic model of the geometry and the results are consistent with previous studies based on cryogenic electron microscopy. The fibrils also give a specific ROA signal, but unlike VCD it is currently not fully explicable by the simulations. In connection with the computational modeling the VOA spectroscopy thus appears as an extremely useful tool for monitoring α-synuclein and other proteins in solutions.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...