CuZrO3: If it exists it should be a sandwich†
Abstract
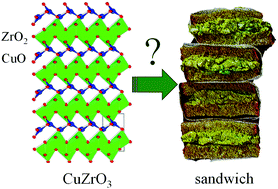
CuZrO3 has been hypothesized to be a catalytic material with potential applications for CO2 reduction. Unfortunately, this material has received limited attention in the literature, and to the best of our knowledge the exact crystal structure is still unknown. To address this challenge, we utilize several different structural prediction techniques in concert, including the Universal Structure Predictor: Evolutionary Xtallography (USPEX), the Materials Project Structure Predictor, and the Open Quantum Materials Database (OQMD). Leveraging these structural prediction techniques in conjunction with Density-Functional Theory (DFT) calculations, we determine a possible structure for CuZrO3, which resembles a “sandwich” morphology. Our calculations reveal that this new structure is significantly lower in energy than a previously hypothesized perovskite structure, albeit it still has a thermodynamic preference to decompose into CuO and ZrO2. In addition, we experimentally tried to synthesize CuZrO3 based on literature reports and compared computational to experimental X-ray Diffraction (XRD) patterns confirming that the final product is a mixture of CuO and ZrO2. Finally, we conducted a computational surface energetics and CO2 adsorption study on our discovered sandwich morphology, demonstrating that CO2 can adsorb and activate on the material. However, these CO2 adsorption results deviate from previously reported results further confirming that the CuZrO3 is a metastable form and may not be experimentally accessible as a well-mixed oxide, since phase segregation to CuO and ZrO2 is preferred. Taken together, our combined computational and experimental study provides evidence that the synthesis of CuZrO3 is extremely difficult and if this oxide exists, it should have a sandwich-like morphology.


 Please wait while we load your content...
Please wait while we load your content...