Super-electrophiles of tri- and tetra-anions stabilized by selected terminal groups and their role in binding noble gas atoms†
Abstract
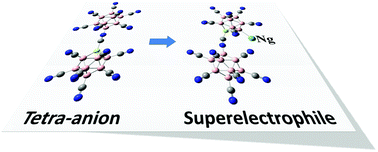
Stabilization of multiply-charged atomic clusters in the gas phase has been a topic of great interest not only because of their potential applications as weakly-coordinating anions, but also for their ability to promote unusual reactions and serve as building blocks of materials. Recent experiments have shown that, after removing one terminal ligand from the closo-dodecacyano-borate, B12(CN)122−, the cluster can strongly bind an argon atom at room temperature. Bearing this in mind, here, we have developed more than a dozen highly stable tri- and tetra-anions using density functional theory (DFT) calculations with hybrid functional (B3LYP) and semi-empirical dispersion corrections. The interactions between the clusters and noble gas atoms, including Ne, Ar and Kr, are studied. The resulting super-electrophilic sites embedded in these charged clusters can bind noble gas atoms with binding energies up to 0.7 eV. This study enriches the database of highly-charged clusters and provides a viable design rule for super-electrophiles that can strongly bind noble gas atoms.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...