The structure of isolated thalidomide as reference for its chirality-dependent biological activity: a laser-ablation rotational study†
Abstract
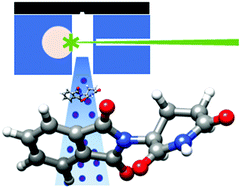
Thalidomide is a drug that presents two enantiomers with markedly different pharmacological and toxicological activities. It is sadly famous due to its teratogenic effects mostly caused by the preferential docking of the (S)-enantiomer to the target protein cereblon (CRBN). To compare the structure of the bound CRBN thalidomide enantiomers with that of the isolated molecule, the rotational spectrum of laser-ablated thalidomide has been studied by chirp-pulsed Fourier transform microwave spectroscopy in supersonic jets complemented by theoretical computations. A new setup of the laser ablation nozzle used is presented. Two stable equatorial and axial conformers of thalidomide have been predicted corresponding to the two possible bent conformations exhibited by the glutarimide moiety. Only the most stable equatorial conformer has been detected. The comparison of its structure with those of the (S)- and (R)-enantiomers bound to CBRN shows that the bound (S) species is only slightly distorted. On the contrary, the bound (R)-enantiomer exhibits a highly distorted structure which affects the degree of puckering of the glutarimide ring and especially to the orientation of the phtalimide and glutarimide subunits. This is consistent with a less stable (R)-enantiomer and the known preference of (S)-thalidomide to bind CRBN, which starts the process leading to teratogenic effects.

 Please wait while we load your content...
Please wait while we load your content...