Heme is responsible for enhanced singlet oxygen deactivation in cytochrome c†
Abstract
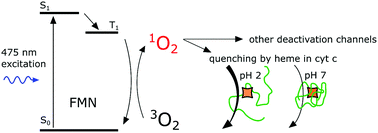
The deactivation of singlet oxygen, the lowest electronic excited state of molecular oxygen, by proteins is usually described through the interaction of singlet oxygen with certain amino acids. Changes in accessibility of these amino acids influence the quenching rate and the phosphorescence kinetics of singlet oxygen. In the cellular environment, however, numerous proteins with covalently bound or encapsulated cofactors are present. These cofactors could also influence the deactivation of singlet oxygen, and these have received little attention. To confront this issue, we used cytochrome c (cyt c) and apocytochrome c (apocyt c) to illustrate how the heme prosthetic group influences the rate constant of singlet oxygen deactivation upon acidic pH-induced conformational change of cyt c. Photo-excited flavin mononucleotide (FMN) was used to produce singlet oxygen. Our data show that the heme group has a significant and measurable effect on singlet oxygen quenching when the heme is exposed to solvents and is therefore more accessible to singlet oxygen. The effect of amino acids and heme accessibility on the FMN triplet state deactivation was also investigated.


 Please wait while we load your content...
Please wait while we load your content...