Structure and thermodynamics of empty clathrate hydrates below the freezing point of water†
Abstract
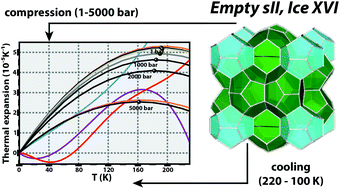
Recently prepared as a new H2O phase, ice XVI was obtained by degassing a Ne-sII clathrate hydrate under vacuum, however very little is known of that crystalline solid under temperatures (T ≤ 220 K) and pressures (p ≤ 5000 bar) relevant for the Earth's environment and geochemistry. In this work, atomically detailed calculations using long time-scale molecular simulations, seldom paralelled before, are employed to probe empty sII clathrate hydrates. It is found that the volumetric response to an applied pressure–temperature gradient is accurately described by the Parsafar and Mason equation of state with an accuracy of at least 99.7%. Structural deformation induced upon the crystals is interpreted by monitoring the unit cell length and isobaric thermal expansivity, whilst benchmarked against previous neutron diffraction measurements of ice XVI and hexagonal ice under room pressure conditions; a critical comparison is established with other sII guest occupied lattices (CH4, CO2 and CnH2n+2 with n = 2, 3, 4), often found in permafrost regions and in the margins of continental shelves. Such an analysis reveals that empty sII frameworks are slightly more stable to thermal deformation than their sI analogues and that hexagonal ice is the structurally most stable of the condensed H2O phases addressed here. Of paramount importance for the oil and natural gas industries, heat capacities obtained from enthalpy profiles are identical for the sI and sII empty clathrates up to 2000 bar and diverge by only ∼7.3% at 5000 bar. The canonical tetrahedral symmetry of water-bonded networks is analysed in terms of an angular and a distance order parameters, which are observed to decrease (increase) as pressure (temperature) increases (decreases). The results now being reported constitute a landmark for future studies dealing with high-pressure and very low-temperature conditions, characteristic of the Earth's permafrost environment and other planetary interiors, whilst contributing to expand our knowledge regarding the recently discovered ice XVI phase.


 Please wait while we load your content...
Please wait while we load your content...