A quantum chemical study on the effects of varying the central metal in extended photosynthetic pigments†
Abstract
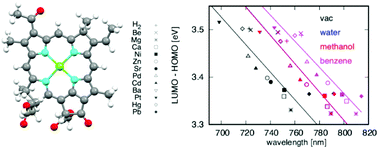
In a certain period of Earth's history, chlorophylls with Mg as their central metal would have been selected as the major photosynthetic pigments, reflecting the radiation in habitats. Assuming evolution in different light and material environments, different photosynthetic pigments would occur. This study is the first attempt to evaluate the physical and chemical properties of model photosynthetic pigments and their potential to function in a variety of light environments using quantum chemistry calculations. Specifically, bacteriochlorophyll b (Bchl b), phthalocyanine (Pht) and meso-dibenzoporphycene (mDBPc) were selected as template molecules, while Be, Mg, Ca, Ni, Zn, Sr, Pd, Cd, Ba, Pt, Hg, Pb and H2 were examined as the central metals in each molecule in various solvents. The results showed that the light absorption by each of these compounds varied over a range of 100 nm depending on the central metal and the surrounding solvent, and Pb produced the largest red shift in the absorption bands of all three photosynthetic pigments. The Pht molecules showed similar redox properties to the chlorophylls, suggesting that these derivatives could be substituted for the special pairs in reaction centers, while the mDBPc molecules appear to be more suitable as accessory pigments due to their extraordinarily broad absorption ranges of approximately 500 nm depending on the conditions.


 Please wait while we load your content...
Please wait while we load your content...