Electromechanically active pair dynamics in a Gd-doped ceria single crystal†
Abstract
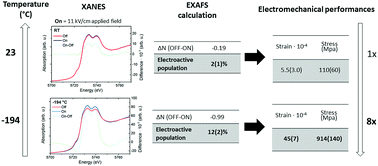
Oxygen-defective ceria, e.g. Gd-doped ceria, shows giant electromechanical properties related to a complex local rearrangement of its lattice. Although they are not entirely identified, the electroactive mechanisms arise from cation and oxygen vacancy (VO) pairs (i.e. Ce–VO), and the local structural elastic distortion in their surroundings. Here, we study the geometry and behaviour of Ce–VO pairs in a grain boundary-free bulk Ce0.9Gd0.1O1.95 single crystal under an AC electric field of ca. 11 kV cm−1. The analysis was carried out through X-ray absorption spectroscopy (XAS) techniques at the Ce L-III edge. Using Density Functional Theory (DFT) calculations, we investigated the effects of the strain on density of states and orbitals at the valence band edge. Our research indicates that electrostriction increases at low temperatures. The electromechanical strain has a structural nature and can rise by one order of magnitude, i.e., from 5 × 10−4 at room temperature to 5 × 10−3 at −193 °C, due to an increase in the population of the electrically active pairs. At a constant VO concentration, the material can thus configure heterogeneous pairs and elastic nanodomains that are either mechanically responsive or not.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...