Superatomic and adsorption properties of Ni atom doped Au clusters†
Abstract
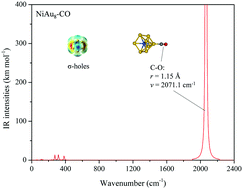
Due to their strong relativistic effects, Au clusters exhibit many unusual geometric structures. Among them, Au7−, Au8 and Au9+ have 18 valence electrons satisfying the magic numbers in the jellium model, respectively, but these three non-spherical clusters are not superatoms. In general, a single dopant atom can drastically change the structural and electronic properties of Au clusters. Here, we searched structures of NiAu7−, NiAu8 and NiAu9+ clusters using the genetic algorithm program (GA) combined with density functional theory (DFT). It was found that the alloy clusters are all 3D spherical structures. The molecular orbitals and density of states analysis indicate that they have completely filled superatomic shells (1S21P6), in which the electronic structure of the Ni atom is d10. Then, the electrostatic potential surfaces of the alloy clusters are analyzed, and the calculated results show that the NiAu8 superatom has remarkable σ-holes with positive potential regions. Moreover, these electron-deficient regions can be considered as interaction sites with some electron donors. After a Lewis base CO gas molecule is adsorbed on the Au-based superatom, we found that the C–O bond distance becomes slightly elongated and its stretching frequency presents a significant red-shift. This is due to the fact that 5d electrons of the Au atom of the NiAu8 transfer towards the anti-bond π orbitals of the CO molecule. Hence, this is an effective strategy for finding new types of superatoms and potential catalysts for covalent bond activation.


 Please wait while we load your content...
Please wait while we load your content...