Understanding the magnetization blocking mechanism in N23−-radical-bridged dilanthanide single-molecule magnets†
Abstract
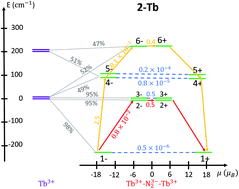
Herein, we report a theoretical investigation of the electronic structure and magnetic properties in [(Cp2Me4HLn(THF))2(μ-N2˙)]− and [(Cp2Me4HLn)2(μ-N2˙)]− (THF = tetrahydrofuran, CpMe4H = tetramethylcyclopentadienyl, Ln = Tb, Dy) complexes [as reported in Demir et al., Nat. Commun., 8, 1–9, 2144 (2017)]. By ab initio methods, their magnetic blocking behaviors are successfully characterized allowing elucidation of the origin of the two blocking barriers observed experimentally. In addition, a detailed analysis of exchange wave functions explains why the blocking barrier of the Tb complexes is roughly twice as large as that of the Dy analogues, a fact which appears to be a general trend exhibited in this family of compounds.
- This article is part of the themed collections: 2021 PCCP HOT Articles and Quantum Computing and Quantum Information Storage


 Please wait while we load your content...
Please wait while we load your content...