Size of the hydrogen bond network in liquid methanol: a quantum cluster equilibrium model with extensive structure search†
Abstract
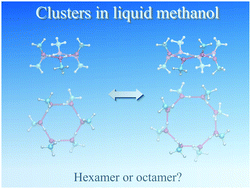
Studies have debated what is a favorable cluster size in liquid methanol. Applications of the quantum cluster equilibrium (QCE) model on a limited set of cluster structures have demonstrated the dominance of cyclic hexamers in liquid methanol. In this study, we examined the aforementioned question by integrating our implementation of QCE with a molecular-dynamics-based structural searching scheme. QCE simulations were performed using a database comprising extensively searched stable conformers of (MeOH)n for n = 2–14, which were optimized by B3LYP/6-31+G(d,p) with and without the dispersion correction. Our analysis indicated that an octamer structure can contribute significantly to cluster probability. By reoptimizing selected conformers with high probability at the MP2 level, we found that the aforementioned octamer became the dominant species due to favorable vibrational free energy, which was attributed to modes of intermolecular vibration.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...