Dipole moment enhanced π–π stacking in fluorophenylacetylenes is carried over from gas-phase dimers to crystal structures propagated through liquid like clusters†‡
Abstract
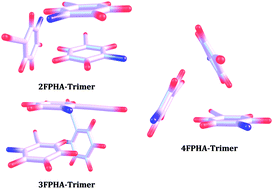
The aggregates of monofluorinated phenylacetylenes in the gas-phase, investigated using the IR-UV double resonance spectroscopic method in combination with extensive structural search and electronic structure calculations, reveal the formation of liquid-like clusters with a π-stacked dimeric core. The structural assignment based on the IR spectra in the acetylenic and aromatic C–H stretching regions suggests that, unlike the parent non-fluorinated phenylacetylene, the substitution of a F atom on the phenyl ring increases the dipole moment, leading to robustness in the formation of a π⋯π stacked dimer, which propagates incorporating C–H⋯π_{Ar/Ac} and C–H⋯F interactions involving both acetylenic and aromatic C–H groups. The structural evolution of fluorophenylacetylene aggregates in the gas phase shows marginal effects due to fluorine atom position on the phenyl ring, with substitution in the para-position tending towards phenylacetylene. The present study signifies that the π⋯π stacked dimers act as a nucleus for the growth of higher clusters to which other molecular units are added predominantly via the {Ar}_C–H⋯π_{Ar} type of interaction and the dominant interactions present in the crystal structures gradually emerge with increasing cluster size. Based on these features, gas-phase clusters of fluorophenylacetylene are hypothesized as “liquid-like clusters” acting as intermediates in the generation of various polymorphic forms starting from a π⋯π stacked dimer as the core molecular unit.


 Please wait while we load your content...
Please wait while we load your content...