Adsorption and reaction pathways of 7-octenoic acid on copper†
Abstract
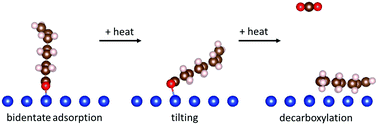
The surface structure and reaction pathways of 7-octenoic acid are studied on a clean copper substrate in ultrahigh vacuum using a combination of reflection–absorption infrared spectroscopy, X-ray photoelectron spectroscopy, temperature-programmed desorption and scanning-tunneling microscopy, supplemented by first-principles density functional theory calculations. 7-Octenoic acid adsorbs molecularly on copper below ∼260 K in a flat-lying configuration at low coverages, becoming more upright as the coverage increases. It deprotonates following adsorption at ∼300 K to form an η2-7-octenoate species. This also lies flat at low coverages, but forms a more vertical self-assembled monolayer as the coverage increases. Heating causes the 7-octenoate species to start to tilt, which produces a small amount of carbon dioxide at ∼550 K and some hydrogen in a peak at ∼615 K ascribed to the reaction of these tilted species. The majority of the decarbonylation occurs at ∼650 K when CO2 and hydrogen evolve simultaneously. Approximately half of the carbon is deposited on the surface as oligomeric species that undergo further dehydrogenation to evolve more hydrogen at ∼740 K. This leaves a carbonaceous layer on the surface, which contains hexagonal motifs connoting the onset of graphitization of the surface.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...