Thioguanine restoration through type I photosensitization-superoxide oxidation-glutathione reduction cycles†
Abstract
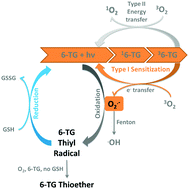
UVA-induced deleterious effect of thiopurine prodrugs including azathioprine, 6-mercaptopurine and 6-thioguanine (6-TG) increases the risk of cancer development due to the incorporation of 6-TG in patients’ DNA. The catalytic mechanism by which thiobases act as a sustained oxidant producer has yet to be explored, especially through the Type I electron transfer pathway that produces superoxide radicals (O2˙−). Under Fenton-like conditions O2˙− radicals convert to extremely reactive hydroxyl radicals (˙OH), thus carrying even higher risk of biological damage than that induced by the well-studied type II reaction. By monitoring 6-TG/UVA-induced photochemistry in mass spectra and superoxide radicals (O2˙−) via nitro blue tetrazolium (NBT) reduction, this work provides two new findings: (1) in the presence of reduced glutathione (GSH), the production of O2˙−via the type I reaction is enhanced 10-fold. 6-TG thiyl radicals are identified as the primary intermediate formed in the reaction of 6-TG with O2˙−. The restoration of 6-TG and concurrent generation of O2˙− occur via a 3-step-cycle: 6-TG type I photosensitization, O2˙− oxidation and GSH reduction. (2) In the absence of GSH, 6-TG thiyl radicals undergo oxygen addition and sulfur dioxide removal to form carbon radicals (C6) which further convert to thioether by reacting with 6-TG molecules. These findings help explain not only thiol-regulation in a biological system but chemoprevention of cancer.


 Please wait while we load your content...
Please wait while we load your content...