Correlating Bromelain's activity with its structure and active-site dynamics and the medium's physical properties in a hydrated deep eutectic solvent†
Abstract
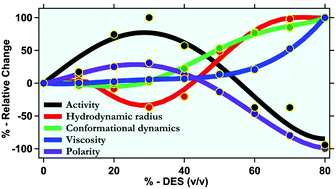
Deep eutectic solvents (DESs) are emerging as new media of choice for biocatalysis due to their environmentally friendly nature, fine-tunability, and potential biocompatibility. This work deciphers the behaviour of bromelain in a ternary DES composed of acetamide, urea, and sorbitol at mole fractions of 0.5, 0.3, and 0.2, respectively (0.5Ac/0.3Ur/0.2Sor), with various degrees of hydration. Bromelain is an essential industrial proteolytic enzyme, and the chosen DES is non-ionic and liquid at room temperature. This provides us with a unique opportunity to contemplate protein behaviour in a non-ionic DES for the very first time. Our results infer that at a low DES concentration (up to 30% V/V DES), bromelain adopts a more compact structural conformation, whereas at higher DES concentrations, it becomes somewhat elongated. The microsecond conformational fluctuation time around the active site of bromelain gradually increases with increasing DES concentration, especially beyond 30% V/V. Interestingly, bromelain retains most of its enzymatic activity in the DES, and at some concentrations, the activity is even higher compared with its native state. Furthermore, we correlate the activity of bromelain with its structure, its active-site dynamics, and the physical properties of the medium. Our results demonstrate that the compact structural conformation and flexibility of the active site of bromelain favour its proteolytic activity. Similarly, a medium with increased polarity and decreased viscosity is favourable for its activity. The presented physical insights into how enzymatic activity depends on the protein structure and dynamics and the physical properties of the medium might provide useful guidelines for the rational design of DESs as biocatalytic media.


 Please wait while we load your content...
Please wait while we load your content...