Tuning the electronic transition energy of indole via substitution: application to identify tryptophan-based chromophores that absorb and emit visible light†
Abstract
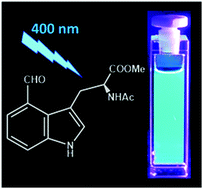
Fluorescent amino acids (FAAs) offer significant advantages over fluorescent proteins in applications where the fluorophore size needs to be limited or minimized. A long-sought goal in biological spectroscopy/microcopy is to develop visible FAAs by modifying the indole ring of tryptophan. Herein, we examine the absorption spectra of a library of 4-substituted indoles and find that the frequency of the absorption maximum correlates linearly with the global electrophilicity index of the substituent. This finding permits us to identify two promising candidates, 4-formyltryptophan (4CHO-Trp) and 4-nitrotryptophan (4NO2-Trp), both of which can be excited by visible light. Further fluorescence measurements indicate that while 4CHO-indole (and 4CHO-Trp) emits cyan fluorescence with a reasonably large quantum yield (ca. 0.22 in ethanol), 4NO2-indole is essentially non-fluorescent, suggesting that 4CHO-Trp (4NO2-Trp) could be useful as a fluorescence reporter (quencher). In addition, we present a simple method for synthesizing 4CHO-Trp.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...