Impurity-induced nematic–isotropic transition of liquid crystals†
Abstract
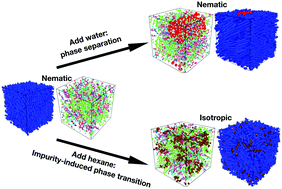
Complex fluids made of liquid crystals (LCs) and small molecules, surfactants, nanoparticles or 1D/2D nanomaterials show novel and interesting features, making them suitable materials for various applications starting from optoelectronics to biosensing. While these additives (impurities) introduce new features in the complex fluids, they may also alter the phase transition behaviour of LCs depending on the physiochemical properties of the added impurity. This article reports on the phase transition of 4-cyano-4′-alkylbiphenyl (nCB) LCs in the presence of an associative impurity, i.e., water and a non-associative impurity, i.e., hexane employing computational methods and experiments. In particular, all-atom (AA) simulations and coarse-grained (CG) models were designed for two complex systems, i.e., 6CB + water and 6CB + hexane and corresponding spectrophotometry experiments were performed using a homologous LC, i.e., 5CB. Results from the simulations and experiments elucidate that the phase transition of LCs depends on the mixing/demixing phenomenon of the impurity in the LC. While associative liquids like water which do not mix with LCs do not influence the nematic-to-isotropic phase transition of LCs, hexane, being a non-associative liquid, mixes well with LCs and induces a sharp impurity-induced nematic-to-isotropic phase transition. Upon application of both AA and CG simulations, we could reach the conclusion that the mixing/demixing phenomenon in an LC + impurity system influences the entropy of the system and hence the observed phase transitions are entropy-driven.


 Please wait while we load your content...
Please wait while we load your content...