Predicting the structure and NMR coupling constant 1J(129Xe–19F) of XeF6 using quantum mechanics methods
Abstract
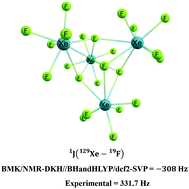
The XeF6 molecule exists as a monomer in the gas phase and as the (XeF6)4 tetramer in solution. Herein we used distinct quantum mechanics methods to study the conformational equilibrium for the XeF6 monomer, which is represented mainly by Oh and C3v symmetric geometries, and for the (XeF6)4 structure found in condensate phases. The NMR 1J(129Xe–19F) coupling constant is predicted using our own NMR-DKH basis set, designed for NMR properties. The C3v conformer of XeF6 was stable only with HF, CCSD, and hybrid DFT functionals with at least 28% exact HF exchange. Increasing the % of HF exchange improves the description of the geometry and the Oh → C3v equilibrium. The BMK, BHandHLYP and LC-ωPBE functionals produce results in excellent agreement with experiments and high-level calculations for the XeF6 molecule. When it comes to the 1J(129Xe–19F) coupling constant, the (XeF6)4 structure must be considered. For that compound, BHandHLYP leads to the best structure, and BMK leads to the best coupling constant; therefore, the generalized protocol BMK/NMR-DKH//BHandHLYP/def2-SVP is recommended to study the XeF6 molecule in the gas phase and solution.


 Please wait while we load your content...
Please wait while we load your content...