Kinetic fall-off behavior for the Cl + Furan-2,5-dione (C4H2O3, maleic anhydride) reaction†
Abstract
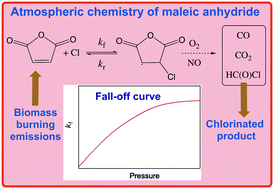
Rate coefficients, k, for the gas-phase Cl + Furan-2,5-dione (C4H2O3, maleic anhydride) reaction were measured over the 15–500 torr (He and N2 bath gas) pressure range at temperatures between 283 and 323 K. Kinetic measurements were performed using pulsed laser photolysis (PLP) to produce Cl atoms and atomic resonance fluorescence (RF) to monitor the Cl atom temporal profile. Complementary relative rate (RR) measurements were performed at 296 K and 620 torr pressure (syn. air) and found to be in good agreement with the absolute measurements. A Troe-type fall-off fit of the temperature and pressure dependence yielded the following rate coefficient parameters: ko(T) = (9.4 ± 0.5) × 10−29 (T/298)−6.3 cm6 molecule−2 s−1, k∞(T) = (3.4 ± 0.5) × 10−11 (T/298)−1.4 cm3 molecule−1 s−1. The formation of a Cl·C4H2O3 adduct intermediate was deduced from the Cl atom temporal profiles and an equilibrium constant, KP(T), for the Cl + C4H2O3 ↔ Cl·C4H2O3 reaction was determined. A third-law analysis yielded ΔH = −15.7 ± 0.4 kcal mol−1 with ΔS = −25.1 cal K−1 mol−1, where ΔS was derived from theoretical calculations at the B3LYP/6-311G(2d,p,d) level. In addition, the rate coefficient for the Cl·C4H2O3 + O2 reaction at 296 K was measured to be (2.83 ± 0.16) × 10−12 cm3 molecule−1 s−1, where the quoted uncertainty is the 2σ fit precision. Stable end-product molar yields of (83 ± 7), (188 ± 10), and (65 ± 10)% were measured for CO, CO2, and HC(O)Cl, respectively, in an air bath gas. An atmospheric degradation mechanism for C4H2O3 is proposed based on the observed product yields and theoretical calculations of ring-opening pathways and activation barrier energies at the CBS-QB3 level of theory.


 Please wait while we load your content...
Please wait while we load your content...