Effect of face-to-face and side-to-side interchain interactions on the electron transport in emeraldine salt polyaniline†
Abstract
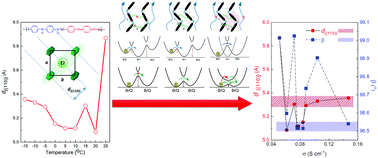
Polyaniline (PANI) is a conductive polymer that has been studied intensively due to its high conductivity, ease of synthesis, fascinating doping mechanism, and a broad spectrum of applications. Polyaniline doped HCl was synthesized by a common direct-oxidation method of aniline using ammonium persulfate as the oxidant in HCl solution at various temperatures. This study focused on conductivity alteration of PANI-ES (emeraldine salt) due to the interchain interaction observed at different reaction temperatures from room temperature down to −15 °C. The molecular structure of PANI-ES was determined by FTIR and Raman spectroscopy. At low reaction temperature, the electronic transport properties improve significantly as reflected by its conductivity. X-ray diffraction (XRD) analysis shows that the value d{(110)} and β play an important role in electron transport through face-to-face and side-to-side interactions, respectively. Scanning electron microscopy (SEM) analysis shows that the morphology of the synthesized PANI-ES consists of granules that are interconnected by nanofibers. Here, the correlation between electronic transport properties, structure, and morphology induced by reaction temperature was analyzed and discussed in detail. Moreover, PANI ES synthesized at 0 °C was applied as an electrocatalytic active layer in the DSSC's counter-electrode with a promising result.


 Please wait while we load your content...
Please wait while we load your content...