Pressure-induced Na–Au compounds with novel structural units and unique charge transfer†
Abstract
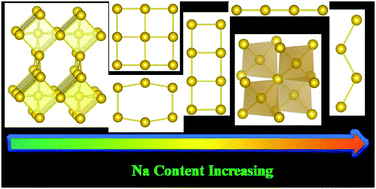
The exploration of novel intermetallic compounds is of great significance for basic research and practical application. Considering the interesting and diverse attributes of Na and Au, their large electronegative difference, and the unresolved high-pressure Na–Au structures, first-principles swarm-intelligence structural search calculations are employed to explore the potential Na–Au compounds at high pressures. Besides reproducing the known Na–Au compounds, eleven new phases are disclosed, exhibiting several unprecedented Au atomic arrangements, such as rectangular ladder, layer formed by edge-sharing squares, hexahedron framework, and diamond-like skeleton, enriching the understanding of Au chemistry. Moreover, the coordination number of Au can be effectively modulated by controlling Na composition. In the Na-rich compounds (Na4Au, Na5Au, and Na6Au), Au shows a formal charge beyond −2, acting as a 6p-block element, originating from pressure-induced unusual Na 3s or 3p → Au 6p charge transfer. These compounds are metallic, but not superconductive. Moreover, the good agreement between the experimental XRD patterns and the simulated ones allows us to assign the predicted P6/mmm Na2Au and Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m Na3Au as the experimental structures at 59.6 GPa. Our work indicates that the modulation of pressure and chemical composition is a useful way to stabilize novel intermetallic compounds.
m Na3Au as the experimental structures at 59.6 GPa. Our work indicates that the modulation of pressure and chemical composition is a useful way to stabilize novel intermetallic compounds.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...