Mechanical hydrolysis imparts self-destruction of water molecules under steric confinement†
Abstract
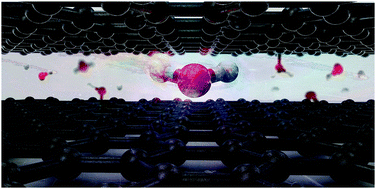
Decoding behavioral aspects associated with the water molecules in confined spaces such as an interlayer space of two-dimensional nanosheets is key for the fundamental understanding of water–matter interactions and identifying unexpected phenomena of water molecules in chemistry and physics. Although numerous studies have been conducted on the behavior of water molecules in confined spaces, their reach stops at the properties of the planar ice-like formation, where van der Waals interactions are the predominant interactions and many questions on the confined space such as the possibility of electron exchange and excitation state remain unsettled. We used density functional theory and reactive molecular dynamics to reveal orbital overlap and induction bonding between water molecules and graphene sheets under much less pressure than graphene fractures. Our study demonstrates high amounts of charge being transferred between water and the graphene sheets, as the interlayer space becomes smaller. As a result, the inner face of the graphene nanosheets is functionalized with hydroxyl and epoxy functional groups while released hydrogen in the form of protons either stays still or traverses a short distance inside the confined space via the Grotthuss mechanism. We found signatures of a new hydrolysis mechanism in the water molecules, i.e. mechanical hydrolysis, presumably responsible for relieving water from extremely confined conditions. This phenomenon where water reacts under extreme confinement by disintegration rather than forming ice-like structures is observed for the first time, illustrating the prospect of treating ultrafine porous nanostructures as a driver for water splitting and material functionalization, potentially impacting the modern design of nanofilters, nanochannels, nano-capacitators, sensors, and so on.


 Please wait while we load your content...
Please wait while we load your content...