Intramolecular charge transfer excitation induced by CH3O substitution in the 3-methoxy-1-propoxy radical†
Abstract
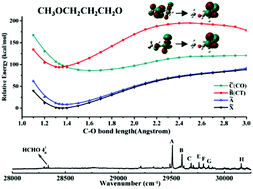
The oxy-substituted alkoxy radicals are generated from the oxidation of ethers. Their degradation path affects ozone production and the formation of the secondary organic aerosol in the atmosphere. In this work, three alkoxy radicals with methoxy (CH3O) substitution at β, γ, and δ carbon are studied using laser-induced fluorescence (LIF) spectroscopy and theoretical calculation methods. A charge transfer (CT) excited state induced by the CH3O substitution is identified to be because of the intramolecular electron transfer from the C–O–C p orbital to the radical O p orbital. Comparison of the structure and CT transition strength between GGt and TTt conformers of the 3-methoxy-1-propoxy radical (CH3OCH2CH2CH2O) suggests that this long-range charge transfer effect is mainly a through-bond interaction. The CT excited state of CH3OCH2CH2CH2O has a conical intersection with the CO σ → O p excited state, which, hence, changes the LIF spectrum of the radical. Only the decomposition product HCHO was observed in the LIF spectrum of β substituted radical CH3OCH2CH2O. For δ substituted radical CH3OCH2CH2CH2CH2O, the substitution effect on the radical stability is negligible and its LIF spectrum is close to that of unsubstituted alkoxy radicals. The results provide information for understanding the degradation chemistry of oxygenated hydrocarbon molecules in the atmosphere.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...