Competition between electron transfer and base-induced elimination mechanisms in the gas-phase reactions of superoxide with alkyl hydroperoxides†
Abstract
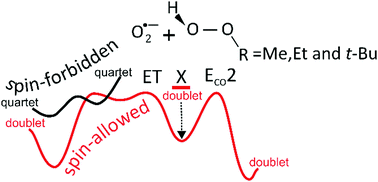
Understanding the mechanism responsible for peroxides decomposition is essential to explain several biochemical processes. The mechanisms of the intrinsic reactions between the superoxide radical anion (O2˙−) and methyl, ethyl, and tert-butyl hydroperoxides (ROOH, with R = Me, Et, and t-Bu) have been characterized to understand the mechanism responsible for peroxides decomposition. The reaction energy diagrams suggest a competition between the spin-allowed and spin-forbidden electron transfer (ET), and base-induced elimination (ECO2) mechanisms. In all cases, the spin-allowed ET mechanism describes formation of the ozonide anion radical (O3˙−), either complexed with an alcohol molecule or separated. For the O2˙−/MeOOH(EtOOH) reactions, HCO2− (MeCO2−) + H2O + HO˙ and OH− + CH2O(MeCHO) + HO2˙ products are associated with the spin-forbidden ET and ECO2 channels, respectively. On the other hand, for the reaction between O2˙− and t-BuOOH, the spin-forbidden ET route describes formation of the MeCOCH2− enolate (either separated or hydrated) along with the methyl peroxyl (MeO2˙) radical. In addition, the regeneration of O2˙−via spin-forbidden ET and ECO2 channels was also characterized from the decomposition of ROOH, yielding diols (CH2(OH)2 and MeCH(OH)2), aldehydes (CH2O and MeCHO), and oxirane (cyc-CH2CMe2O).
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...